3 Subatomic Particles That Make Up an Atom
Atoms make up everything that has massthat is everything that takes up space. The majority of an atoms mass comes from the protons and neutrons that make up its nucleus.
The Famous Types Of Subatomic Particles Praxilabs
Terms in this set 3 Which two subatomic particles make up the nucleus of an atom.

. The value of difference in electronegativity between two atoms in a covalent bond is less than 17. Which part of an atom has the most mass. What did Ernest Rutherfords gold-foil experiment show.
The number of protons is always equal to number of. Protons neutrons and electrons. The particles are held within the atom by four fundamental forces- gravity electromagnetic force strong force and weak force.
An atom itself is made up of three tiny kinds of particles called subatomic particles. What subatomic particles make up an atom. Protons neutrons 0 electrons - There are more than three in most everything accept Deuterium 1 of each.
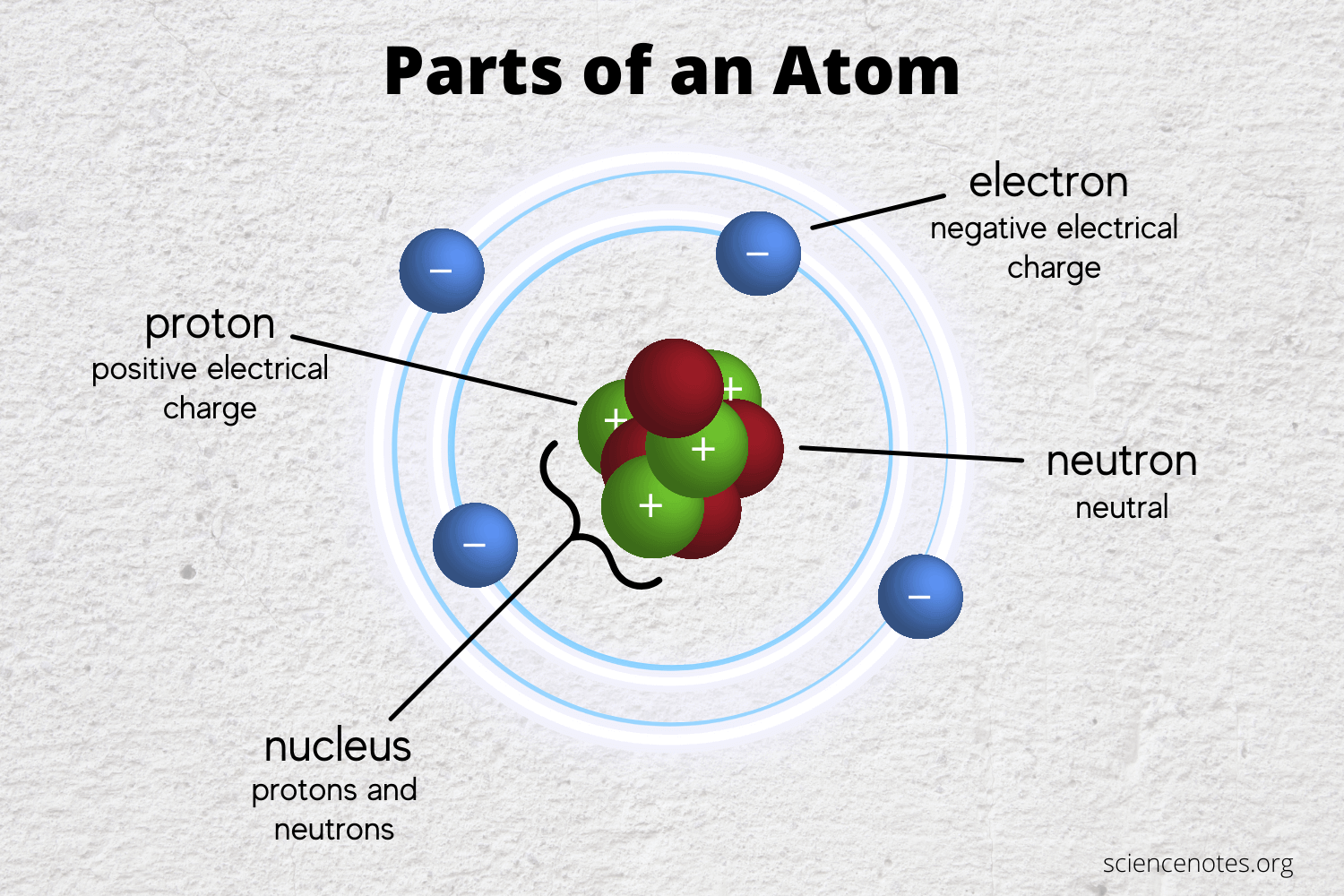
Protons and neutrons An atom has 2 protons in its nucleus. There are three subatomic particles called electrons protons and neutrons. Protons have a positive charge while electrons have a negative charge.
The atom has 2 negatively charged electrons outside the nucleus. Helium has 6 and Hydrogen 2 no neutron in its most common form. Electrons are the least massive of an atoms constituent particles with a mass of 911 x 10 31 kg and a size too small to be measured by current techniques.
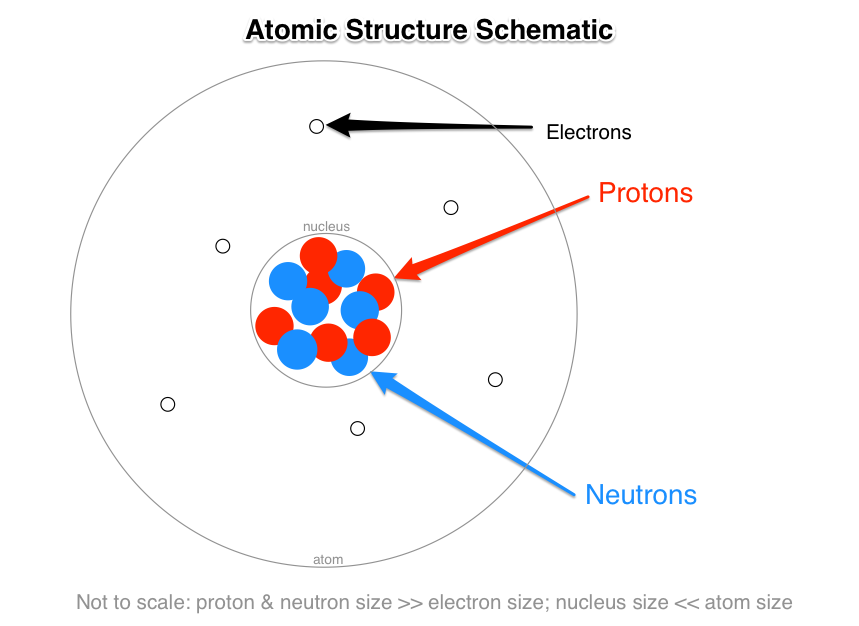
The three subatomic particles that make up the atom are protons and neutrons inside the nucleus of the atom and electrons which are located in the electron cloud surrounding the atomic nucleus. The three sub-atomic particles that make up the atom are. Use the data to answer the following questions all questions refer to p d 0.
What subatomic particles make up an atom. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud. What are 3 subatomic particles that make up an atom.
Leave a Reply Cancel reply. If you have to name three subatomic particles of an atom they are protons electrons and neutrons. Required fields are marked Comment Name Email.
There are three subatomic particles. 3 main subatomic particles that form the atom Protons neutrons and electrons At the center of the atom is the Nucleus Covalent bonds form when two atoms have a very small nearly insignificant difference in electronegativity. Which subatomic particles makes up most of the mass of the atom.
Two of the subatomic particles have electrical charges. Your email address will not be published. Answer 1 of 3.
3 Show answers Another question on Chemistry. Lem 2 the data below are for the system ethyl propyl ether 1-chloroform 2 at 05 bar. Protons are the positively charged particles electrons.
Which statement must be true for this atom to have no net electrical charge. Electrons have a negative charge protons have a positive charge neutrons have no charge. Name the three subatomic particles of an atom.
Give the difference between three subatomic particles. Protons neutrons and electrons.

The Chemistry Of Life Objectives What Three Subatomic Particles Make Up Atoms How Are All The Isotopes Of An Element Similar What Are The Two Types Ppt Download
What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic

Atomic Structure Nucleus Contains Protons And Neutrons Matter Science Relative Atomic Mass Atomic Structure
No comments for "3 Subatomic Particles That Make Up an Atom"
Post a Comment